Drosophila Neuroscience
Synaptic Plasticity

Model system
The fly neuromuscular junction (NMJ) is an excellent system to study synapses and their plasticity. A single neuron, with multiple attachments to the muscle (boutons) that functions almost exactly like mammalian synapses do in the brain. The morphology of the larval insect allows us to have access to intact synapses for imaging, chemical stimulation and electrophysiology all while maintaining normal cellular function.
Memories are made of this
This model of synaptic connections allows us to study the cellular, sub-cellular and physiological processes involved in new synapse growth. As synaptic growth is so central to long-term memory formation, this readily accessible system is an extremely powerful tool for studying key questions in this field.
Seeing is believing
The single-neuron makeup of the NMJ system means that we can visualise the role RNA has to play in synaptic plasticity to great effect. Both fixed and live imaging of single molecules can be achieved with widefield, confocal and super-resolution microscopy methods.
Selected Synaptic Plasticity Papers
(2023) Gala DS, Titlow JS, Teodoro RO, Davis I. Far from home: the role of glial mRNA localization in synaptic plasticity. RNA.
(2020) Titlow J, Robertson F, Järvelin A, Ish-Horowicz D, Smith C, Gratton E, Davis I.
Syncrip/hnRNP Q is required for activity-induced Msp300/Nesprin-1 expression and new synapse formation. J Cell Biol.
(2018) Titlow JS, Yang L, Parton RM, Palanca A, Davis I.
Super-Resolution Single Molecule FISH at the Drosophila Neuromuscular Junction. Methods Mol Biol.
(2014) McDermott SM, Yang L, Halstead JM, Hamilton RS, Meignin C, Davis I.
Drosophila Syncrip modulates the expression of mRNAs encoding key synaptic proteins required for morphology at the neuromuscular junction. RNA.
Neural Stem Cell Development
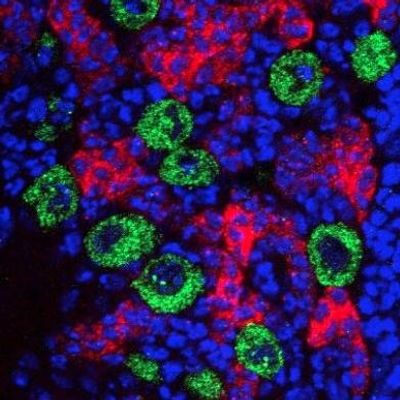
Fly Brains
The human brain is incredibly complex, consisting of billions of neural and glial cells. Drosophila brains have approximately 100,000 neurons, and yet retain many of the same functions and modes of action as the human brain. This makes the fly brain a tractable and simpler system to study and understand while maintaining all of same cellular functions as mammalian brains.
Neuroblasts
All of the tens of thousands of cells in the fly brain arise from a small number of neural stem cells, or neuroblasts. These cells divide over and over to generate the cells needed for the brain until they eventually stop being stem cells and turn into neurons. In the Davis lab we are interested in how RNA and RNA-binding proteins interact to determine the fate of neuroblasts, impacting on how and when the change from stem cell to neuron is controlled.
Intact Brains Under the Microscope
A real advantage of fly brains is our ability to dissect them out of the insect intact and image them without sectioning. Whole mount brains imaged both fixed and live give us insight into biologically relevant detail across the entire organ.
Selected Publications
(2023) Thompson MK, Ceccarelli A, Ish-Horowicz D, Davis I. Dynamically regulated transcription factors are encoded by highly unstable mRNAs in the Drosophila larval brain. RNA.
(2020) Samuels TJ, Järvelin AI, Ish-Horowicz D, Davis I.
Imp/IGF2BP levels modulate individual neural stem cell growth and division through myc mRNA stability. Elife.
(2020) Hailstone M, Waithe D, Samuels TJ, Yang L, Costello I, Arava Y, Robertson E, Parton RM, Davis I.
CytoCensus, mapping cell identity and division in tissues and organs using machine learning. Elife.
(2020) Samuels TJ, Arava Y, Järvelin AI, Robertson F, Lee JY, Yang L, Yang CP, Lee T, Ish-Horowicz D, Davis I.
Neuronal upregulation of Prospero protein is driven by alternative mRNA polyadenylation and Syncrip-mediated mRNA stabilisation. Biol Open.
(2017) Yang CP, Samuels TJ, Huang Y, Yang L, Ish-Horowicz D, Davis I, Lee T.
Imp and Syp RNA-binding proteins govern decommissioning of Drosophila neural stem cells. Development.
smFISH Screen

Lots of Genes, Single Molecules
A large-scale screen of the Drosophila nervous system where the protein and RNA of hundreds of genes have been screened. The RNA of each gene has been screened with single molecule resolution, giving a wealth of data about how genes in the nervous system are regulated.
Post-transcriptional regulation
Much of molecular biology research has focussed on control of gene transcription. In the Davis lab we are interested in RNA and how post-transcriptional regulation is involved in so many biological systems. This screen has opened up a treasure trove of questions we can pursue in the lab
Displaying the data
The large-scale nature of imaging screens such as this makes them difficult to browse and make connections. In order to help us integrate our imaging and metadata in a manageable and explorable way we have collaborated with MDV to develop an interactive collection for users to navigate.
Publications
(2023) Titlow JS, Kiourlappou M, Palanca A, Lee JY, Gala DS, Ennis D, Yu JJS, Young FL, Susano Pinto DM, Garforth S, Francis HS, Strivens F, Mulvey H, Dallman-Porter A, Thornton S, Arman D, Millard MJ, Järvelin AI, Thompson MK, Sargent M, Kounatidis I, Parton RM, Taylor S, Davis I. Systematic analysis of YFP traps reveals common mRNA/protein discordance in neural tissues. J Cell Biol.
(2017) Yang L, Titlow J, Ennis D, Smith C, Mitchell J, Young FL, Waddell S, Ish-Horowicz D, Davis I.
Single molecule fluorescence in situ hybridisation for quantitating post-transcriptional regulation in Drosophila brains. Methods.
(2018) Titlow JS, Yang L, Parton RM, Palanca A, Davis I. Super-Resolution Single Molecule FISH at the Drosophila Neuromuscular Junction. Methods Mol Biol.
Data Management and Image Analysis

Data Data Everywhere
Modern scientific methods can generate truly huge datasets. Managing and analysing these datasets properly can be extremely difficult using traditional methods. In the Davis lab we work with collaborators and within the lab to develop tools to help with these types of datasets.
Image Management
Modern imaging systems generate high quality images with large file sizes. When movies, time-lapses and screening projects (see above) are part of your data it can quickly encompass hundreds of files making up many terabytes of data. In order to catalogue, store and curate this data we work with the Open Microscopy Environment, OMERO. This collaborative open-source system allows us to manage our large datasets in a user-friendly way.
CytoCensus
Using machine learning tools has allowed us to study highly complicated organs such as the Drosophila brain and monitor growth and cell divisions over a period of several days. This open-source software can be adapted to many systems and uses a minimal user-input method to train the software to identify cells of interest. These can then be tracked through live imaging of the tissue of interest. All of the code and user manuals can be found on Github.

